Our Growth Drivers
Innovative Science & Business Models
Our Growth Drivers
Innovative Science & Business Models
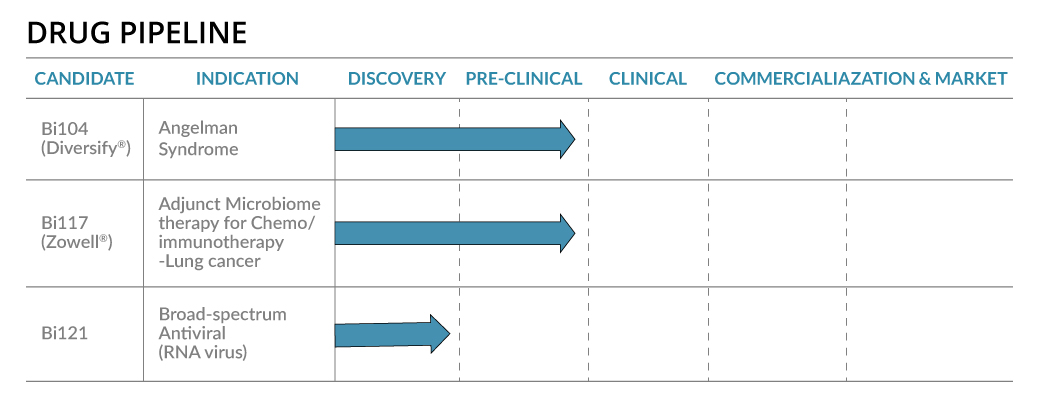
Bi121- Broad Spectrum Antiviral (Patent-pending)
The SARS-CoV-2 pandemic infected 343 million people with over 5.59 million deaths. New mutated lineages of SARS-CoV-2 such as Omicron are evolving faster. Broad-spectrum viral inhibitors that block the initial stage of infection by reducing virus proliferation and disease severity is an unmet global medical need. We studied Bi121, a standardized polyphenolic-rich compound isolated from Pelargonium sideodes, against recombinant Vesicular Stomatitis Virus (rVSV)-pseudotyped SARS-CoV-2S (spike) that represent mutations in the spike protein of six different variants of SARS-CoV-2. Bi121 was effective in neutralizing all six rVSV-ΔG-SARS-CoV-2S variants expressing different mutations. The antiviral activity of Bi121 was then assessed against three variants of SARS-CoV-2 (USA WA1/2020, Hongkong/VM20001061/2020, B.1.167.2 (Delta)) using RT-qPCR and plaque assays in two different cell lines (Vero cells and HEK-ACE2). Bi121 showed significant activity toward all the three variants tested, suggesting a broad-spectrum activity
Publication:
VaxxiBiTM– Novel solid Adjuvant (Patent-pending)
Biom Pharmaceutical has developed a patent pending stable nanoemulsion-adjuvant formulation called VaxxiBi and conducting preliminary studies on the efficacy of this system. The suppleness and lateral mobility of VaxxiBi, which is an antigen-loaded in solid-particle based nanoemulsions, may provide a facile, effective, safe and broadly applicable strategy to enhance adaptive immunity against infections and diseases. Preliminary studies on the shows that VaxxiBi provides an increased CD4+/CD8+ T-cell response to the one or more antigens compared to a composition in the absence of the said adjuvant